|
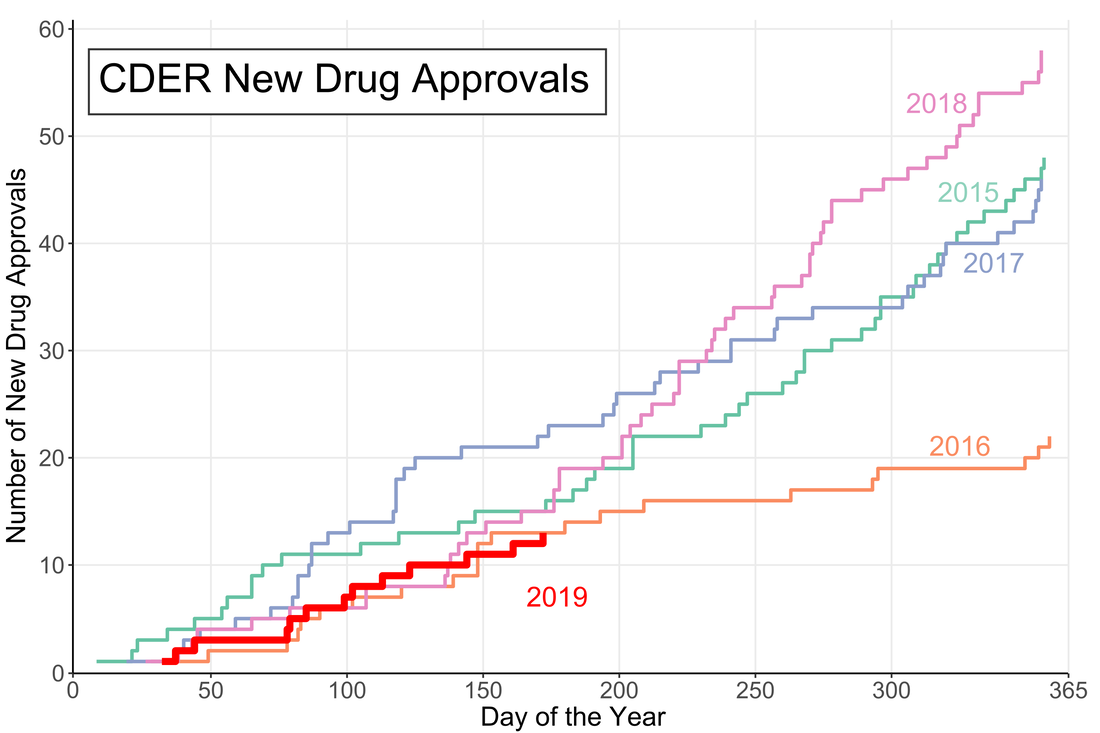
A little over halfway through the year and the US Food and Drug Administration (FDA) appears to be on track for either a big year of new drug approvals or....not. The number of new molecular entities (NMEs) approved by FDA's Center for Drug Evaluation and Research (CDER) are equal to the number approved at this point of the year in 2016 and only two product apporvals behind both 2018 and 2015. Despite starting the year off with the longest federal shutdown in history the FDA is keeping pace with past years.
However, the figure demonstrates another important fact: approval numbers mid-year do not correlate strongly with year-end approvals. While the number of approvals were similar in 2016 and 2018, the end year totals were wildly different. In 2018, CDER approved a record 59 NMEs while 2016 approved less than half of that number. Additionally, in 2017, the number of NME approvals at mid-year was much higher than any other year, but finished in line with the number of approvals in 2015 and well below the number of approvals in 2018. It seems that the future could go either way. There could be a dramatic up-tic in CDER approval rate as in 2018 (perhaps from shutdown-delayed applications) or the rate could slow to a crawl like in 2016.
0 Comments
Leave a Reply. |
Archives
July 2023
Categories
All
|
 RSS Feed
RSS Feed